Wu Tingyao


As the mood of the whole mankind fluctuates with the “COVID-19 vaccine” and “COVID-19 pandemic”, hand-foot-and-mouth disease, common in children under 5 years old, has also entered a high epidemic period. Its symptoms usually include fever, mouth sores, and skin rash commonly found on the hands, mouth, and/or feet. This disease is actually caused by enterovirus infection.
There are many types of enteroviruses, most of which are mild and can be cleared up by the immune system within a week.Some enteroviruses can be very severe, causing serious neurological complications such as meningitis, myelitis, acute flaccid paralysis, and even death.
Enterovirus A71, referred to as EV-A71, is this kind of evil that needs to be guarded carefully. Just as the COVID-19 vaccine is needed to prevent the novel coronavirus, vaccination is undoubtedly the best way to prevent EV-A71 infection.
However, as everyone knows, no vaccine is 100% effective. How to improve vaccine efficacy?
The research published in Frontiers in Immunology, the official journal of the International Union of Immunological Societies in September 2020 by the Graduate Institute of Immunology, National Taiwan University College of Medicine, and the Department of Medical Research, National Taiwan University Hospital confirmed that:
The EV-A71 vaccine added with Ganoderma lucidum polysaccharides can promote the production of neutralizing antibodies, induce anti-virus-related immune responses, suppress the amount of virus in animals, prevent the occurrence of severe illness (invasion of the central nervous system causing acute flaccid paralysis) and reduces the risk of death.
The EV-A71 vaccine used in the experiment is an inactivated vaccine made of the inactivated (dead) virus. Different from traditional injections, this vaccine is designed as nasal drops, which are directly dropped into the nostrils and absorbed through the mucous membrane to induce the production of antibodies.
As for the Ganoderma lucidum polysaccharide added to the vaccine as an adjuvant to “enhance the vaccine-induced immune response”, it is derived from the hot water extract of Ganoderma lucidum fruiting bodies, which is referred to as PS-G (polysaccharide purified from Ganoderma lucidum) in this research.
In animal experiments, healthy mice were inoculated with 12 μL vaccine drops from their nostrils. The mice were divided into three groups according to the difference in vaccinations:
◆ RD lysate Group (No Vaccine Group): the placebo vaccine is vaccinated, which can be regarded as the representative of no vaccination;
◆ EV-A71 Group (Vaccine Group): the vaccinated vaccine contains 2.5 μg of inactivated EV-A71;
◆ EV-A71+PS-G Group: the vaccinated vaccine contains 2.5 μg of inactivated EV-A71 plus 20 μg polysaccharide purified from Ganoderma lucidum (PS-G).
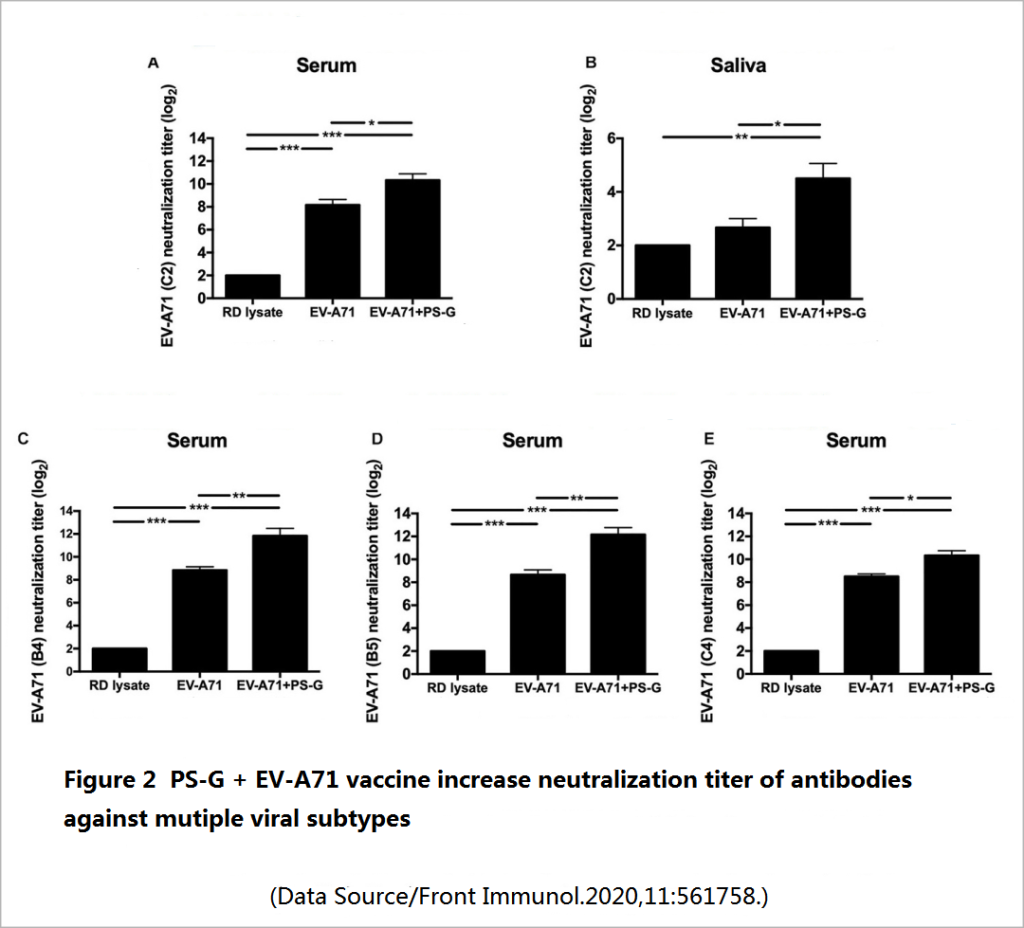
Two weeks after the mice completed the three doses of vaccination, the level of long-term effective antibodies against EV71 in the mice was tested ─- including the IgG that mainly exists in serum and body fluids, and the IgA that is second in serum content and mainly resides in mucosal tissues (oral mucosa, nasal mucosa, respiratory mucosa, and intestinal mucosa). It was found that compared with a simple vaccine, a vaccine supplemented with PS-G can promote the production of more specific IgG and IgA antibodies (Figure 1), and these antibodies have a good neutralization effect on various genetic variants of EV71 subtype, and also have a stronger inhibitory effect on the virus-infected cells (Figure 2).
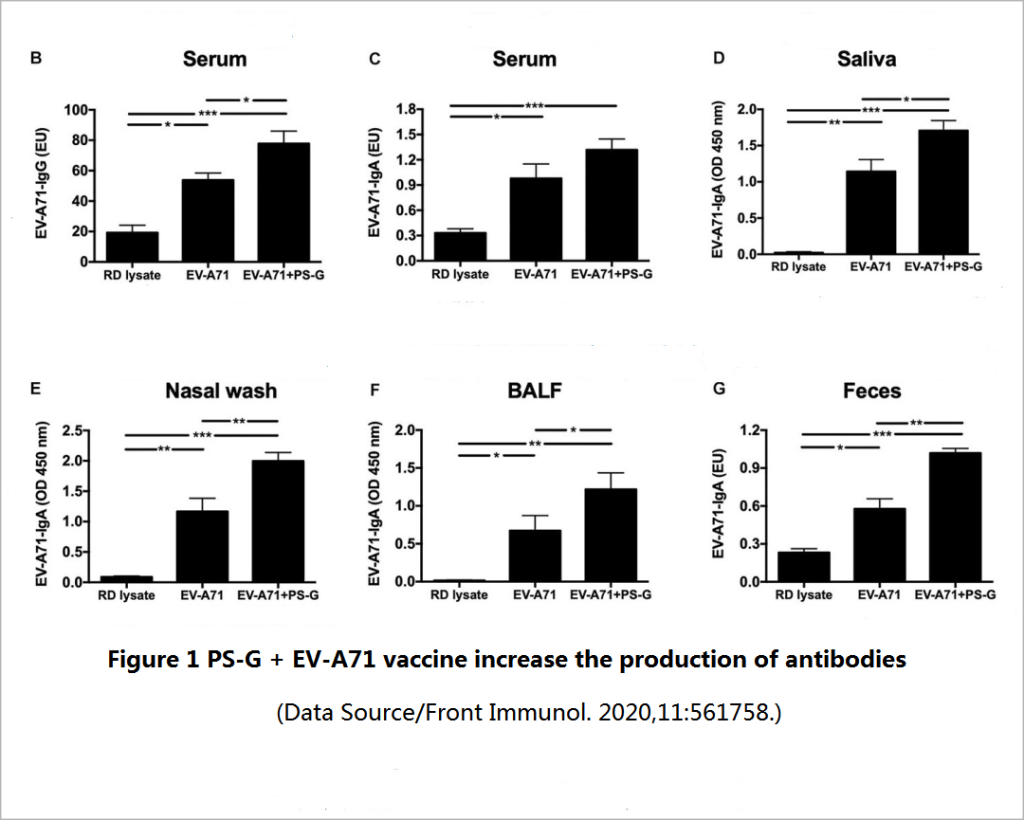
A good vaccine should be able to not only induce the production of effective antibodies but also induce the production of “memory B cells” so that as soon as the virus invades, it can start to secrete antibodies to supplement fresh troops for the frontline without spending time waiting for T cell instructions.
At the same time, a good vaccine should also help T cells keep in mind the appearance of the virus so that T cells can respond quickly and lead the entire immune corps to fight the virus more efficiently. Regarding this part, the EV71 vaccine added with PS-G is indeed more potent than the vaccine without PS-G. It not only doubles the memory B cells in the spleen of mice (Figure 3) but also promotes the spleen and intestinal lymphatic tissue to secrete more anti-viral T cells and related cytokines-interferon (IFN-γ) and interleukin 17 (IL-17) when it fights hand to hand with the virus.
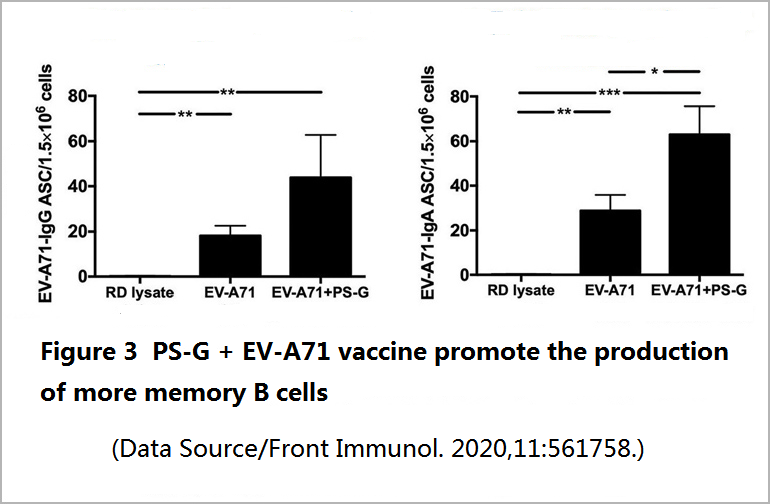
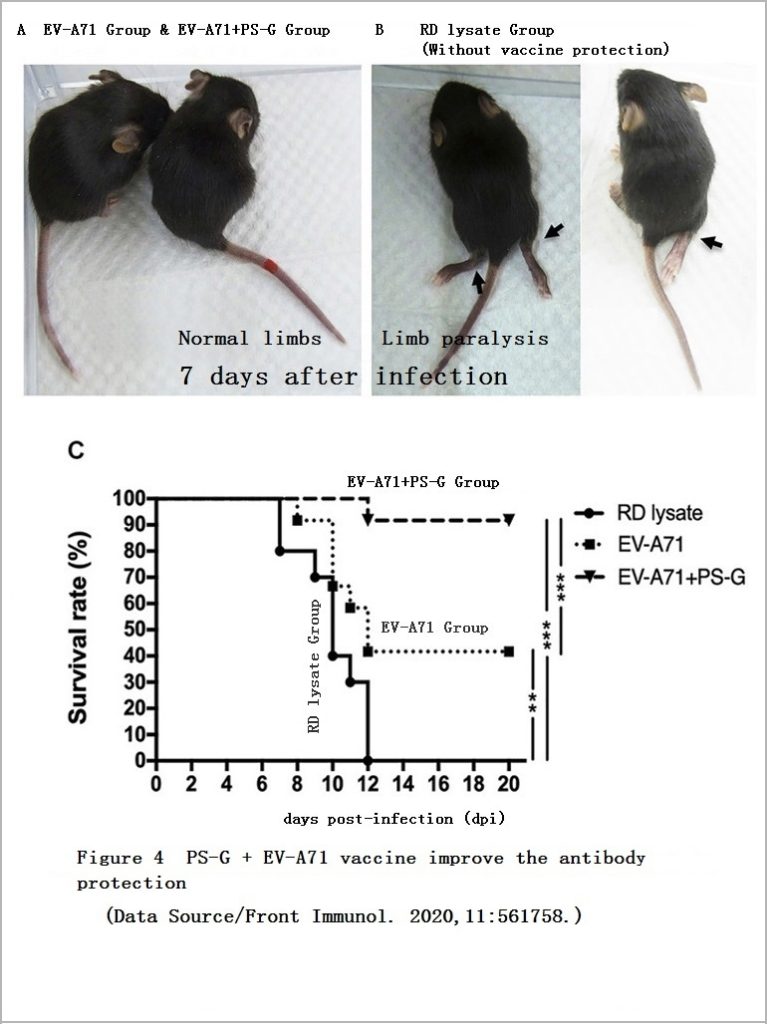
In order to better understand the protective effect of the vaccine, the researchers did two animal experiments. One of the experiments was to inject the serum of the vaccinated mice and EV71 into the young mice with defective B cells for observation for 20 days. The less challenged the young mice were by the virus, the more effective the vaccine-induced antibodies were.
It was found that no matter whether the young mice received serum from the “EV-A71″ Group or the “EV-A71+PS-G” Group, symptoms such as limb paralysis could be avoided. However, the young mice that received serum from the “EV-A71+PS-G” Group had a less amount of virus in the central nervous system, and the mortality rate is also lower, which means that the antibodies induced by the vaccine added with PS-G have better ability to inhibit virus reproduction and provide higher protection (Figure 4, Figure 5).
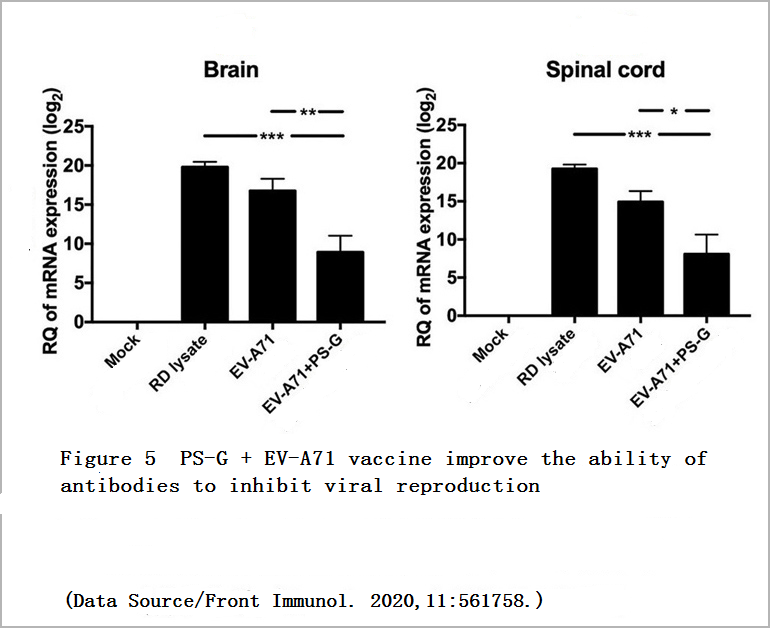
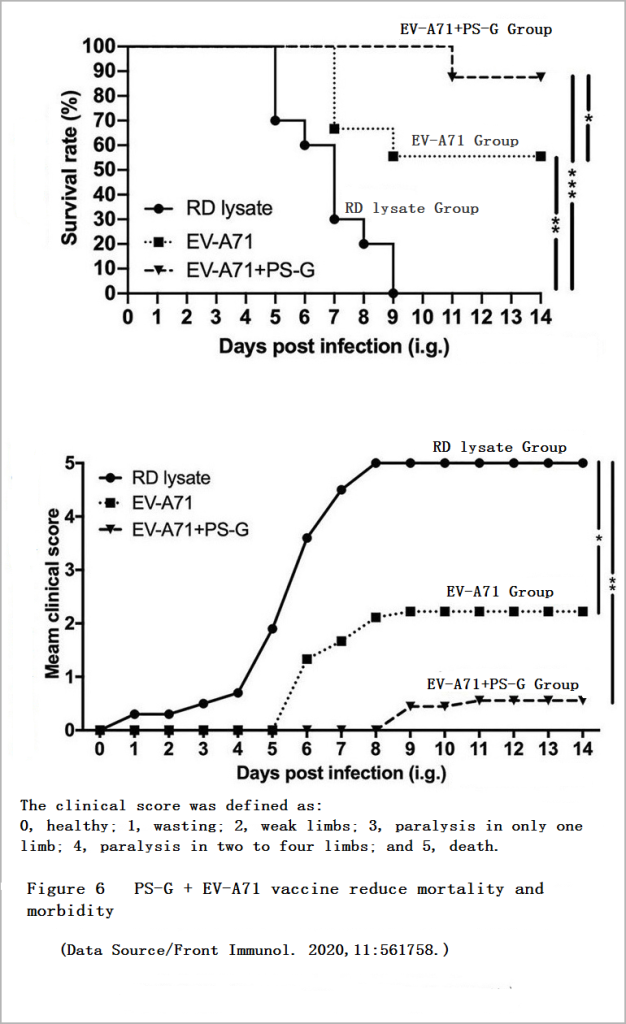
In another animal experiment, the researchers used young mice with normal immune functions but easily infected with EV71. These young mice received a dose of the vaccine on the 7th and 14th days after birth. The researchers then sent EV71 into the stomachs of these young mice through the oral cavity of these young mice to induce infection on the 21st day to observe the effect of the vaccine on inducing the young mice’s own immune response.
After two weeks, the survival rate of young mice vaccinated with “PS-G + vaccine” was 89%, and the survival rate of young mice vaccinated with “vaccine” was 56%, and the overall health of the former was significantly better than that of the latter (Figure 6), PS-G obviously has a nonnegligible significance in enhancing the protective efficacy of vaccines.
The role of vaccine adjuvants is to initiate an immune response, prompting the immune system to accurately identify the antigens (such as viruses) in the vaccine and produce better protective efficacy while safety is the biggest prerequisite for choosing an adjuvant. From the above research results, it can be seen that the PS-G can double the original immune effect of the vaccine, which provides a valuable reference for how to improve the protective efficacy of the vaccine.
The active polysaccharide from the fruiting body of Ganoderma lucidum has been proven in the past to improve the identification and elimination of invaders by the immune system through macrophages, dendritic cells, T cells, B cells… This is why it works well as a vaccine adjuvant. We are unlikely to encounter a vaccine containing PS-G, but before vaccination, using Ganoderma lucidum to boost immunity is something we can decide and do.
[Data Source]
Yu-Li Lin, et al. A Polysaccharide Purified From Ganoderma lucidum Acts as a Potent Mucosal Adjuvant That Promotes Protective Immunity Against the Lethal Challenge With Enterovirus A71. Front Immunol. 2020,11:561758. doi: 10.3389/fimmu.2020.561758.
END
About the author/ Ms. Wu Tingyao
Wu Tingyao has been reporting on first-hand Ganoderma lucidum information
since 1999. She is the author of Healing with Ganoderma (published in The People’s Medical Publishing House in April 2017).
★ This article is published under the exclusive authorization of the author, and the ownership belongs to GANOHERB
★ The above works cannot be reproduced, excerpted or used in other ways without the authorization of GanoHerb
★ If the works have been authorized to be used, they should be used within the scope of authorization and indicate the source: GanoHerb
★ Violation of the above statement, GanoHerb will pursue its related legal responsibilities
★ The original text of this article was written in Chinese by Wu Tingyao and translated into English by Alfred Liu. If there is any discrepancy between the translation (English) and the original (Chinese), the original Chinese shall prevail. If readers have any questions, please contact the original author, Ms. Wu Tingyao.




