8月 26, 2015 / 九州大学 / 科学レポート
文/呉廷耀
The research team of Kuniyoshi Shimizu, 九州大学農学研究所准教授, それを確認した 31 霊芝の子実体から単離されたトリテルペノイドは、5 種類のインフルエンザ A ウイルスのノイラミニダーゼをさまざまな程度で阻害します。, among which there are two triterpenoids even suitable for development as anti-influenza drugs. The research results were published in “Scientific Reports” under the “Nature” publishing group at the end of August 2015.
Neuraminidase is one of the two proteins protruding on the surface of influenza A viruses. Each influenza virus has about one hundred of these proteases. When the virus invades the cell and uses the material in the cell to replicate new virus particles, neuraminidase is needed for the new virus particles to break away from the cell and further infect other cells. したがって, when neuraminidase loses its activity, the new virus will be locked in the cell and cannot escape, the threat to the host will be reduced, and the disease can be controlled. The commonly used oseltamivir (Tamiflu) in clinical practice is to use this principle to prevent the proliferation and spread of the virus.
According to research conducted by Kuniyoshi Shimizu, at a concentration of 200 μM, these Ganoderma triterpenoids inhibited the activity of H1N1, H5N1, H7N9 and two resistant mutant strains NA (H1N1, N295S) and NA (H3N2, E119V) to varying degrees. 概して, the inhibitory effect on neuraminidase of N1 type (especially H5N1) is the best, and the inhibitory effect on neuraminidase of H7N9 is the worst. Among these triterpenoids, ganoderic acid TQ and ganoderic acid TR showed the highest levels of inhibition, and the effects of these two compounds ranged from 55.4% に 96.5% inhibition for different NA subtypes.
Further analysis of the structure-activity relationship of these triterpenoids revealed that the triterpenoids, which have a better inhibitory effect on N1 neuraminidase, have the main structure of “tetracyclic triterpenoids with two double bonds, a branch as a carboxylic group, and an oxygen-containing group at the R5 site” (Backbone A in the figure below). If the main structure is the other two (Backbone B and C in the figure below), the effect will be poor.
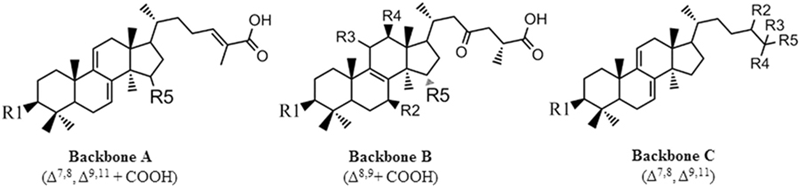
(Source/Sci Rep. 2015 8月 26;5:13194.)
In silico docking is used to simulate the interaction of ganoderic acids (T-Q and TR) and neuraminidases (H1N1 and H5N1). 結果として, it was found that both ganoderic acids and Tamiflu were able to directly bind to the active area of neuraminidase. This active area is composed of several amino acid residues. Ganoderma acids TQ and TR will bind to the two amino acid residues Arg292 and Glu119. Tamiflu has another option but can also make neuraminidase ineffective.
Compared with inhibiting other proteins on the influenza virus (such as the M2 protein, which opens the virus shell at the moment the virus binds to the host cell and sends the viral genes to the cell), neuraminidase inhibitors are currently recognized as effective and less resistant influenza treatment drugs. したがって, researchers believe that ganoderic acids T-Q and TR, which are similar to but not the same in the mechanism of Tamiflu, have the opportunity to be used as a new generation of anti-influenza drugs or design references.
しかし, there is a prerequisite for the drug to be used as an anti-influenza drug, つまり, the drug must effectively inhibit the reproduction of the virus without harming the cells infected by the virus. しかし, in experiments on cells infected with live viruses and breast cancer cell lines (MCF-7), it was found that when the researchers used these two types of ganoderic acids alone, they had doubts about high cytotoxicity, but they also found another kind of Ganoderma triterpenoid, ganoderol B, has an inhibitory effect on H5N1 (but the inhibitory effect is poor), but it is not cytotoxic. したがって, researchers believe that how to improve the safety of ganoderic acids T-Q and TR through modification of the chemical structure while still retaining their inhibition of neuraminidase activity must be carefully considered.
[ソース] Zhu Q, 他. Inhibition of neuraminidase by Ganoderma triterpenoids and implications for neuraminidase inhibitor design. 科学担当者. 2015 8月 26;5:13194. 土肥: 10.1038/srep13194.
終わり
著者について/Mさん. 呉廷耀
ウー・ティンヤオ氏は、以来、霊芝に関する直接の情報を報告し続けている。 1999. 彼女はの著者です 霊芝による治癒 (4月に人民医学出版社に出版 2017).
★この記事は著者の独占的な許可を得て掲載されています.
★上記作品は転載禁止です, 著者の許可なく抜粋または他の方法で使用される.
★上記記載事項に違反した場合, 著者は関連する法的責任を追及します.
★この記事の原文はWu Tingyaoが中国語で執筆し、Alfred Liuが英語に翻訳しました。. 翻訳に齟齬があった場合 (英語) そしてオリジナル (中国語), 本来の中国人が勝つだろう. 読者に質問がある場合, 原作者に連絡してください, MS. 呉廷耀.



