
С смягчением правил управления, конкуренция среди однородных продуктов Рейши неизбежно станет более напряженной. Почему, казалось бы, одинаковые товары различаются по цене? Потому что детали определяют качество и ценность. Все законы приводят к одному и тому же выводу, и то же самое с Рейши.
С 1970-х годов, the success of artificial cultivation ofРейши and the progress of extraction technology have opened up a large amount of modern scientific research on Reishi, providing a basis for Reishi’s high safety and numerous effects, driving the vitality of the Reishi industry, promoting the development of Reishi products, and also inspiring people’s confidence and enthusiasm for eating Reishi. However, as Reishi gradually becomes a health shield for modern people, various controversies continue to be rumored.
People with the same disease takeРейши, some find it effective, others do not; some find it effective this time, but not the next; some experience side effects or worsening of the disease after taking it, while others improve all the way and even other conditions are improved; some have been eating for decades and are getting healthier, while others have red indicators for liver and kidney function; even scientists are baffled, because they all use Reishi for research and the experimental methods are the same, but the results of this time are vastly different from the last time or the results previously published by others…
Such contradictions and confusions aboutРейши are not exclusive to a specific region, group of people, or product, but are a collective common experience. The main reasons are: Mistakenly believing that Reishi can be distinguished from its appearance; Mistakenly believing that all Reishi are more or less the same; And mistakenly believing that anything that can be called Reishi must necessarily match the beautiful evaluations of Reishi in ancient books, and must necessarily possess the various effects of Reishi proven by science.
The crux of these misconceptions about Reishi is summarized as follows:
1. Whether it isРейши cannot be determined by the naked eye.
Фактически, “looking like Reishi” and “being Reishi” are not equivalent! Whether a mushroom can be scientifically classified as “Reishi”, and whether it can bear the surname “Ganoderma” in the first word of the Latin scientific name, depends on its spores, то есть, the reproductive cells of Reishi, whether they have the exclusive feature of Reishi spores being “asymmetrically ovoid and having a double cell wall”.
IncludingРейши, many mushrooms are easily affected by the external environment during their growth process. The final appearance may be typical or atypical. When someone uses what looks like but is actually “inconsistent inside and out”, they naturally cannot expect the safety and efficacy of Reishi; the wish to take it for a long time like an immortal to lighten the body and prolong life may naturally be replaced by “becoming an immortal”.
2. Reishi is not just one kind, and the composition of ingredients varies.
Moreover, Reishi is not just one kind, but there are many kinds (here “kind” means species, corresponding to the English “species”). These different kinds of Reishi, although the first word of the Latin scientific name is all “Ganoderma”, are essentially different from each other like chickens and ducks, and cannot mate to breed the next generation.
While chickens, утки, and even geese can be distinguished by the naked eye, different kinds of Reishi are hard to distinguish from their appearance because their morphological appearance is easily affected by the environment. Since different kinds ofРейши have different ingredient composition characteristics, when your Reishi raw materials use this kind today and that kind tomorrow, even if the processing method is the same, the final product will naturally have many problems in terms of composition and efficacy.
3. Variedingredientsлead tovariedеffects.
Рейши, often associated with longevity and vitality since ancient times, has been scientifically proven to have numerous health benefits such as tumor inhibition, immune regulation, inflammation alleviation, blood pressure reduction, lipid and glucose level reduction, антивозрастные свойства, защита печени, and heart health improvement, among others. However, these effects are attributed to different ingredients found in Reishi. Therefore, not all products labeled as Reishi can deliver all of its known benefits. The specific effects depend on the ingredients present in the product and whether their concentrations are sufficient to be effective.
The composition ofРейши is influenced by many factors. The strain of the mushroom is just the starting point. Even different varieties (also known as strains) derived from the same strain can have different compositions (the concept of “strain” to “variety” is akin to “dog” to “Chihuahua”, “Husky”). Более того, the types and concentrations of Reishi ingredients can vary due to a multitude of factors such as growth environment (latitude, влажность, temperature, свет, air circulation), cultivation conditions (Duanwood, nutrients, moisture), harvest timing, processing procedures, and extraction techniques. This intricate interplay of factors underscores the complexity of Reishi’s composition.
4. Unpollutedcultivationеnsures thesafety ofРейши.
Compared to the natural purity of ancient times, the modern environment is filled with various forms of pollution. Therefore, even if what is being cultivated is “real Reishi”, whether the final product can be as “non-toxic” as stated in ancient books, or as proven by scientific toxicology tests, where experimental animals were given large amounts of Reishi extract for three consecutive months (almost like eatingРейши as a meal and in very filling quantities), with no negative impact on the animals’ survival, appearance, масса, behavior, blood biochemical indicators, and important organs (сердце, печень, селезенка, легкие, kidneys, мозг, intestines), it also depends on the growth environment of Reishi.
This is also why wildРейши should not be harvested, because even if you guessed correctly, the risk of toxicity is very high. However, even artificially cultivated Reishi may not necessarily be safe, because if the cultivator has not done a good job of verification, and used pesticide-contaminated, heavy metal-contaminated, microorganism-contaminated logs, nutrients, water sources, soil… during the cultivation process, the Reishi(including spore powder) that is grown cannot possibly let people rest easy.

Conditions for the Safety and Efficacy of Reishi
Therefore, the safety and efficacy of Reishi are conditional and premised on the following:
1. The source of the strain or variety is clear and remains consistent throughout;
2. The base itself and the surrounding environment are free from pollution;
3. Выращивание, harvesting, обработка, добыча, and other operational processes need to be standardized and controlled;
4. Pesticides, тяжелые металлы, and harmful microorganisms related to product safety need to be monitored;
5. Active ingredients related to product quality need to be tested.
Imagine, if the raw materials of Reishi are purchased from everywhere, the sources of the raw materials are mixed and uneven, no matter how high-end the subsequent processing, добыча, packaging, and marketing are, it will be of no avail. Not to mention achieving a product that matches its name and maintains the original quality assurance!
Therefore, if a company wants to produce a safe and effectiveРейши продукт, even if it does not have its own research and development center to develop exclusive varieties for expert identification, it should at least purchase legally identified strains or varieties from specialized institutions for cultivation. Moreover, it must have its own cultivation base, production workshop, testing equipment, talent training, and traceability management to possibly provide stable reproducible conditions for the safety and efficacy of Reishi. These “self-requirements” hidden in the details naturally become part of the cost.
Detailsdeterminequality andvalue.
С смягчением правил управления, Рейши, which could only appear in the past as health foods, ломтики, and drugs that have passed efficacy certification, can now also be used as food ingredients (implemented from November 9, 2023). The health effect of Reishi single-formula health food claiming to “help enhance immunity” will also become easier (the record system will be implemented from May 1, 2024).
The future market will be more dazzling than it is now, and the price competition of homogeneous goods will inevitably be more intense. When consumers face more and more choices, they must remember the truth that “details determine quality and value”.
Рейши is included in the “Catalog of Substances that are Traditionally Both Food and Chinese Medicinal Materials”, which means that Reishi can be used as a food ingredient, or made into general food for sale, or added to general food for sale. However, the premise is that the heavy metal content (lead, кадмий, мышьяк, Меркурий) of Reishi raw materials must be under the safety limit of the national standard for food safety.
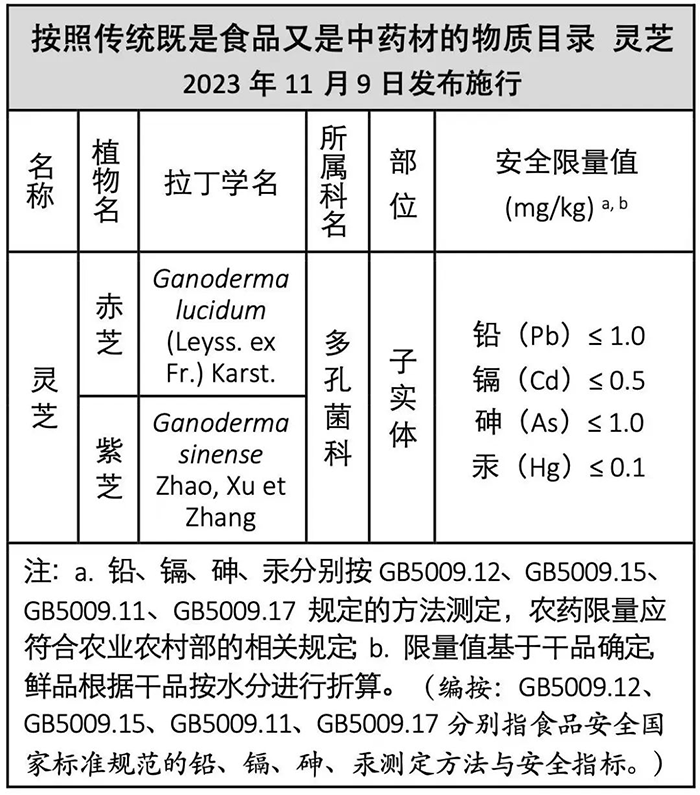
The content of this table is compiled from official announcements (referenced in this article as Reference 1). If there are discrepancies, please refer to the official announcements.
The inclusion of Reishi in the “Health Food Raw Material Directory” means that after the single-formula health food made from Reishi raw materials is recorded (registered), it can claim the health effect of “helping to enhance immunity” within the prescribed dosage range. There is no need for verification of safety and efficacy by a third-party impartial unit, nor is there a need to submit experimental results for national approval. However, the premise is that the raw materials must comply with the current version of the “Chinese Pharmacopoeia” for Reishi, включая:
Use the dried fruiting body ofГанодерма яркая илиГанодерма китайская, where the moisture content must not exceed 17.0%, the total ash content must not exceed 3.2%, the content of Reishi polysaccharides (calculated as anhydrous glucose) must not be less than 0.9%, and the content of triterpenes and sterols (calculated as oleanolic acid) must not be less than 0.5%. It needs to be stored in a dry place to prevent mold, moth damage, and similar issues.
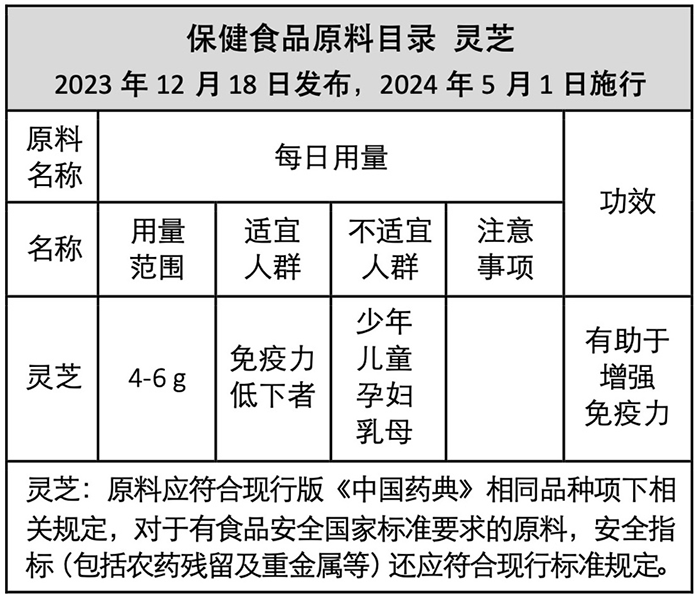
The content of this table is compiled from official announcements (referenced in this article as Reference 2). If there are discrepancies, please refer to the official announcements.
If we look back at the earlier regulations related to the inclusion of cell-wall brokenПорошок спор Рейши in the “Health Food Raw Material Directory”, we will find that the spore powder that can be claimed to “help enhance immunity” actually has to meet many requirements. These include: source of raw materials (Reishi strains), quality level (sterilization, drying, cell-wall breaking method, скорость разрушения клеточной стенки, color, вкус, powder state, moisture and ash content, oxidation situation), безопасность (heavy metal and microbial indicators), and active ingredient content (polysaccharides ≥0.9%), и т. д.. The number of requirements is even more than that for the fruiting body.
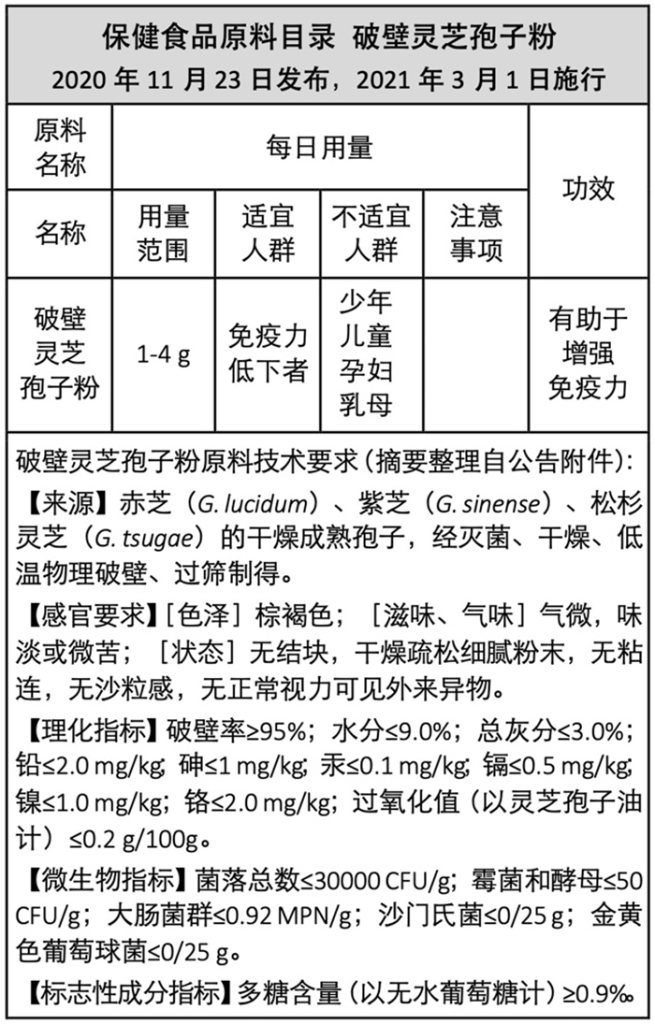
The content of this table is compiled from official announcements (referenced in this article as Reference 4). If there are discrepancies, please refer to the official announcements.
Not allРейши in the world are the same.
Given the various regulations mentioned above regarding quality, безопасность, and ingredients, it means that the safety and effectiveness of Reishi are not automatically satisfied by just bearing the name “Reishi”, but are subject to many conditions. It also indicates that there will be unsafe and ineffective Reishi, whether it is due to inadvertence or short-sightedness.
To ensure that every batch ofРейши raw materials and every batch of Reishi products meet the compliance standards and are true to their name, and not mislead consumers into the misunderstanding of Reishi, it requires a lot of investment from practitioners in professionalism, management, equipment, and talent, as well as the most important conscience. Because making Reishi is not difficult to say, not simple to say, it mainly depends on whether it is done well.
Those who have been maintaining their health with real Reishi and good Reishi for a long time will definitely have a profound experience of “fortunately, there is Reishi” in the course of life when they “slowly get old” or when their “health suddenly flashes a red light”. Although health is priceless, Reishi has a price. As for whether it is worth the price, the best judgment basis is not only the credible reputation, the long-term accumulated brand reputation, but also personally seeing how the Reishi you want to use is grown and made.
The more virtuous the company, the more open and honest it is, and the more it welcomes consumers to explore. If you want to see it with your own eyes, but the manufacturer does not want you to see the growth environment of Reishi in every possible way, and even makes hisРейши mysterious, what “level” do you think such Reishi will be?»

Ссылки
1. Announcement (Нет. 9 из 2023) by the National Health Commission and the State Administration for Market Regulation on the inclusion of nine substances such as Codonopsis pilosula that are traditionally both food and Chinese medicinal materials, released on November 9, 2023.
2. Announcement (Нет. 57 из 2023) by the State Administration for Market Regulation, the National Health Commission, and the National Administration of Traditional Chinese Medicine on the release of the directory of three health food raw materials including ginseng, released on December 18, 2023.
3. Compiled by the National Pharmacopoeia Committee. Линчжи. Фармакопея Китайской Народной Республики (2020 Edition, Vol. я, пп. 201-202). Published by China Medical Science and Technology Press on May 1, 2020.
4. Announcement (Нет. 54 из 2020) by the State Administration for Market Regulation, the National Health Commission, and the National Administration of Traditional Chinese Medicine on the release of the directory of five health food raw materials including Coenzyme Q10, released on November 23, 2020.
КОНЕЦ

★ Данная статья разрешена к публикации исключительно автором., и его право собственности принадлежит GanoHerb.
★ Воспроизведение вышеуказанных работ не допускается., извлечено или использовано другими способами без разрешения GanoHerb.
★ Лица, получившие право на использование произведений, должны использовать их в пределах разрешения и указать источник.: ГаноХерб.
★ GanoHerb будет нести юридическую ответственность против тех, кто нарушает вышеуказанное заявление..
★ Оригинал рукописи этой статьи был написан на китайском языке У Тинъяо и впоследствии переведен на английский Альфредом Лю.. В случае любых несоответствий между английским переводом и оригинальным китайским текстом, Последний должен иметь приоритет. Для любых запросов, пожалуйста, обратитесь к первоначальному автору, РС. У Тинъяо.



